Carbon dioxide (CO₂) is vital for animals and plants on land and in the water. Many marine organisms, such as algae and plankton utilise CO₂ for photosynthesis. They convert carbon dioxide and sunlight into energy and oxygen. These organisms are at the beginning of the food chain and serve as a food source for many other marine animals.
CO₂ also dissolves in seawater and is stored in form of carbonic acid. If the ocean absorbs a lot of the carbon dioxide released by humans from the atmosphere, it leads to fundamental changes in the marine chemistry.
The acidity of the sea
What exactly happens to the CO2 in the water? The answer is: carbonates are used during carbon dioxide binding in the surface water and so-called hydrogen cations (protons) are released under certain circumstances. The number of free hydrogen cations in turn determines the acidity of the seawater. The greater their number, the more acid the water contains.
The concentration of hydrogen cations in a solution is measured using the so-called pH value. It indicates how acidic or alkaline a liquid is. The pH scale ranges from 0 (very acidic) to 14 (very alkaline). This means the more hydrogen cations there are in a solution, the lower the pH value.
The average pH value of the sea surface has fallen from 8.2 to 8.1 since the beginning of industrialisation. This supposedly small step on the logarithmic pH scale corresponds to a real increase in acidity of 26 per cent - a change that the world's oceans and their inhabitants have not experienced in millions of years.
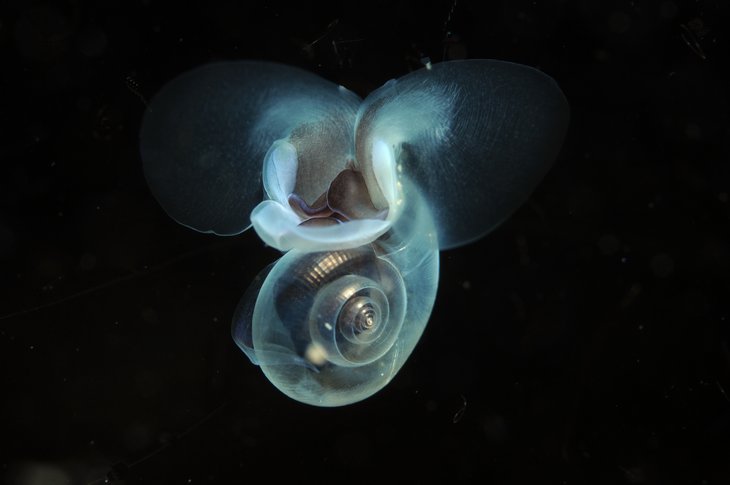
The increasing acidification of the oceans has an impact on various biological processes, and therefore on the lives of many marine organisms. The decreasing availability of carbonates makes it more difficult for calcifying organisms such as corals, mussels, winged snails and chambered molluscs to build their calcareous shells or skeletons. They become thinner and more fragile. We know that echinoderms such as sea urchins and starfish grow less and die earlier with increasing acidification. Fish are particularly at risk at an embryonic or larvae stage. In both early stages of development, the animals do not yet have a functioning acid-base regulation.
It is also unclear to what extent the various marine organisms can adapt to ocean acidification. Single-celled algae and small zooplankton with short reproductive cycles appear to be better equipped than larger organisms with long reproductive cycles.
CO₂ is therefore vital for marine organisms, however, an imbalance caused by too much CO₂ in the water can severely disrupt the marine ecosystem.
- Woods Hole Oceanographic Instituion (WHOI) (o.D.). Know Your Ocean. Ocean Topics - Ocean Acidification. www.whoi.edu/know-your-ocean/ocean-topics/how-the-ocean-works/ocean-chemistry/ocean-acidification/